Novel Systemic and Biologic Agents for Atopic Dermatitis
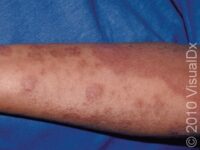
Atopic dermatitis, more commonly known as eczema, is a common chronic skin condition caused by the immune system attacking the skin. Eczema can significantly impact a patient’s quality of life and interfere with common daily activities. The negative impact of eczema on patients is primarily due to the debilitating itch or skin pain associated with this condition. Historically, the treatment of eczema was primarily managed by preventive measures (eg, avoiding allergens or other environmental factors that irritate the skin), the daily use of moisturizing products, or the application of topical medications such as over-the-counter or prescription-strength steroids. These preventive or topical therapies; however, are often insufficient for eczema patients with widespread or severe skin disease.
In the last decade, several new therapies have emerged for the treatment of eczema. These medications hold great potential for effectively managing widespread forms of eczema. This article will explore several approved medical treatments for eczema.
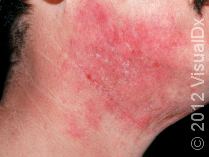
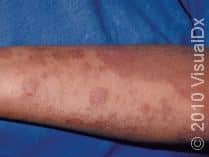
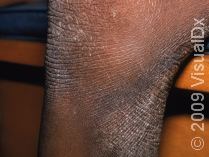
What Treatments Are Usually Tried First for Eczema?
First-line treatments for eczema include daily moisturizers and topical anti-inflammatory agents (eg, topical steroids or other nonsteroidal topical medications, such as calcineurin or PDE4 inhibitors). Phototherapy, commonly referred to as light therapy, is also used for patients with eczema that is not responsive to topical treatments or is more widespread on the body. However, light therapy can be expensive and time-consuming as it typically requires 2-3 treatments a week as well as an insurance copay for each light treatment. Additionally, prolonged exposure to specific light therapies has been associated with an increased risk of skin cancer, and light treatments can be less effective in patients with darker skin colors.
What Is a Systemic Agent or Biologic Therapy and When Are They Used for Eczema?
Systemic agents are a class of medical treatments that work throughout the body. They are typically given orally or as a subcutaneous injection (an injection under the skin). Injectable medications are referred to as biologic medications. Oral or biologic systemic medications are typically preferred for eczema patients with widespread skin disease, eczema not responsive to topical or light-based treatments, or the treatment of disease involving sensitive or special sites (eg, the face, genitals, or palms / soles), where the use of topical medications may be ineffective or inappropriate. A systemic treatment may also be preferred in patients with eczema symptoms that significantly affect an individual’s daily activities or sleep. Several systemic agents are now available for the treatment of eczema.
How Do Newer Systemic or Biologic Medications Agents Work?
Recent advances in medical treatments for eczema have introduced promising biologic medications such as dupilumab (Dupixent) and tralokinumab (Adbry), as well as oral systemic treatments like upadacitinib (Rinvoq) and abrocitinib (Cibinqo). These medications are specifically designed to target and block the primary signals from the immune system (eg, cytokines or interleukins) that are responsible for the disease manifestations and symptoms of eczema.
Dupilumab (Dupixent) is a biologic that helps block the primary sources of inflammation in eczema. Specifically, this medication blocks the inflammatory effects of 2 immune proteins called interleukin-4 and -13 (IL-4 and IL-13), which contribute to the skin findings seen in eczema. It is self-administered via a subcutaneous injection twice monthly for most patients. It is currently US Food and Drug Administration (FDA) approved for the treatment of eczema that is inadequately controlled by topical treatments in adults and children ages 6 months or older. It is also FDA approved for several other chronic inflammatory conditions, including asthma, eosinophilic esophagitis, prurigo nodularis, and chronic rhinosinusitis with nasal polyposis.
Tralokinumab (Adbry) is another biologic medication approved for the treatment of eczema in adults. This medication works very similarly to dupilumab, but it only blocks IL-13 of the immune response. Like dupilumab, it is a self-administered subcutaneous injection given every 2 weeks.
Are Dupilumab and Tralokinumab Safe to Use?
Based on extensive clinical trials, we know that both medications are highly effective for the treatment of eczema with very few side effects. Patients receiving dupilumab and tralokinumab may experience mild conjunctivitis (irritation of the clear covering of the eye), which presents as eye redness or itching. However, most patients never experience these uncommon eye symptoms, or the eye symptoms may only occur once and resolve despite ongoing injections. Additionally, both new biologic medications do not interact with common oral medications that patients may be taking for other medical conditions, such as elevated blood pressure, high cholesterol, or diabetes.
How do Dupilumab and Tralokinumab Compare to Oral Systemic Medications like Rinvoq or Cibinqo?
Dupilumab and tralokinumab are targeted medications that act like snipers against the predominant immune signals causing eczema (ie, they decrease a few key signals causing eczema while leaving the rest of the immune system relatively unaffected). In contrast, upadacitinib and abrocitinib (commonly referred to as JAK inhibitors) are less specific than a biologic and impact many more immune signals contributing to eczema. This broader action can be very useful for patients with severe eczema, especially those who did not respond to topical medications, light therapy, or biologic treatment with dupilumab or tralokinumab.
Upadacitinib and abrocitinib are oral medications rather than skin injections. Upadacitinib is approved for the treatment of eczema in patients who are 12 years or older, whereas abrocitinib is only approved for use in adult patients. However, the broader action of these 2 medications results in a greater suppression of the immune system’s protective response and results in an increased risk of side effects, such as infections, blood clots, heart issues, and possibly cancer. For this reason, JAK inhibitors such as upadacitinib and abrocitinib should be used with caution and under the close supervision of an experienced dermatologist familiar with eczema treatments.
How Do You Decide Which Systemic Agent to Use?
When considering a biologic or oral systemic medication for eczema, several factors should be considered. These factors include the severity of eczema, the body sites affected, prior treatments tried, age, and preference for a specific administration method (eg, topical, oral pill, or skin injection). Biologic and oral medications are also expensive when not covered by health insurance, so the preferred medication by your insurance provider often determines which medication your health care professional can choose.
Conclusion
The ability to treat eczema has dramatically improved in the last decade. There are now multiple highly effective oral and injectable treatments for difficult-to-treat or severe eczema. Nevertheless, choosing the right medication for your eczema can be deeply personal and sometimes overwhelming. Therefore, finding a medical professional such as a dermatologist who can help you understand your options and select the best treatment for your eczema is essential.
References
Bieber T, Simpson EL, Silverberg JI, et al; JADE COMPARE Investigators. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021 03 25;384(12):1101-1112. PubMedId: 33761207.
Paller AS, Siegfried EC, Cork MJ, et al. Laboratory safety from a randomized 16-week phase III study of dupilumab in children aged 6 months to 5 years with moderate-to-severe atopic dermatitis. Paediatr Drugs. 2023 Jan;25(1):67-77. PubMedId: 36529811.
Reich K, Thyssen JP, Blauvelt A, et al. Efficacy and safety of abrocitinib versus dupilumab in adults with moderate-to-severe atopic dermatitis: A randomised, double-blind, multicentre phase 3 trial. Lancet. 2022 Jul 23;400(10348):273-282. PubMedId: 35871814.
Simpson EL, Merola JF, Silverberg JI, et al. Safety of tralokinumab in adult patients with moderate-to-severe atopic dermatitis: Pooled analysis of five randomized, double-blind, placebo-controlled phase II and phase III trials. Br J Dermatol. 2022 Dec;187(6):888-899. PubMedId: 36082590.
Wollenberg A, Blauvelt A, Guttman-Yassky E, et al; ECZTRA 1 and ECZTRA 2 study investigators. Tralokinumab for moderate-to-severe atopic dermatitis: Results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br J Dermatol. 2021 03;184(3):437-449. PubMedId: 33000465.
Last modified on October 13th, 2023 at 10:46 am